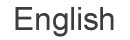腫瘍細胞研究部 Department of Cancer Cell Research
メンバー
水平方向にスクロールできます。
| 部長(PI) | 山口 英樹 | Hideki Yamaguchi | Researchmap/Google Scholar メールアドレス h-yamaguchi |
| 研究員 | Krishnaraj Jayaraman | Krishnaraj Jayaraman | |
| 研究助手 | 永村 ゆう子 | Yuko Nagamura | 研究事務室 職員兼任 |
| 客員研究員 | 宮崎 允 | Makoto Miyazaki | 千葉県がんセンター研究所 Researchmap |
| 客員研究員 | 清野 透 | Tohru Kiyono | Researchmap |
| 客員研究員 | 土屋 紅緒 | Benio Tsuchiya | 杏雲堂病院病理部兼任 Researchmap |
| 協力研究員 | 松永 深里 | Misato Matsunaga | 星薬科大学薬学部 |
| 協力研究員 | 山川 穂華 | Honoka Yamakawa | 星薬科大学薬学部 |
| 協力研究員 | 大崎 睦生 | Mutsuki Osaki | 東京バイオテクノロジー専門学校 |
※メールアドレスには@po.kyoundo.jpをつけて下さい
研究内容
がんは日本人の死亡原因の1位であり、がん死のおよそ 9割が転移によると考えられています。従って転移の制御は、がん治療において最も重要な課題の1つです。しかし転移の分子機構には未だ不明な部分が多く、治療法の開発にはさらなる生物学的解析が必要だと考えられます。当研究部では、がん細胞が転移する際に働く浸潤突起と呼ばれる細胞構造の形成機構と機能を解明すること、また日本人に多い難治がんであるスキルス胃がんが腹腔内組織に転移する機序を解明することを目的として研究を行っています。本研究で得られる成果は、がん転移の分子機構の解明と、転移を標的とした全く新しいがん治療法の開発につながるものと期待されます。① 浸潤突起形成の分子機構と血行性転移における機能解析
浸潤突起は、浸潤性がん細胞により形成される膜構造であり、アクチン繊維と細胞外基質分解酵素に富み、細胞外基質を分解する活性を持っています(図1)。私たちはこれまでに多くの浸潤突起構成・制御分子を同定してその機能を明らかにしてきました。また乳がんの血行性転移において、浸潤突起が重要な役割を果たすことを示しました。現在も新規制御分子を複数見いだしており、浸潤突起形成を制御する分子メカニズムの解析を行なっています。さらに浸潤突起形成を指標としたハイスループットスクリーニング実験系を用いて、遺伝子ライブラリーや化合物ライブラリー等のスクリーニングを行うことにより、新規機能分子や阻害薬候補化合物の探索を進めています。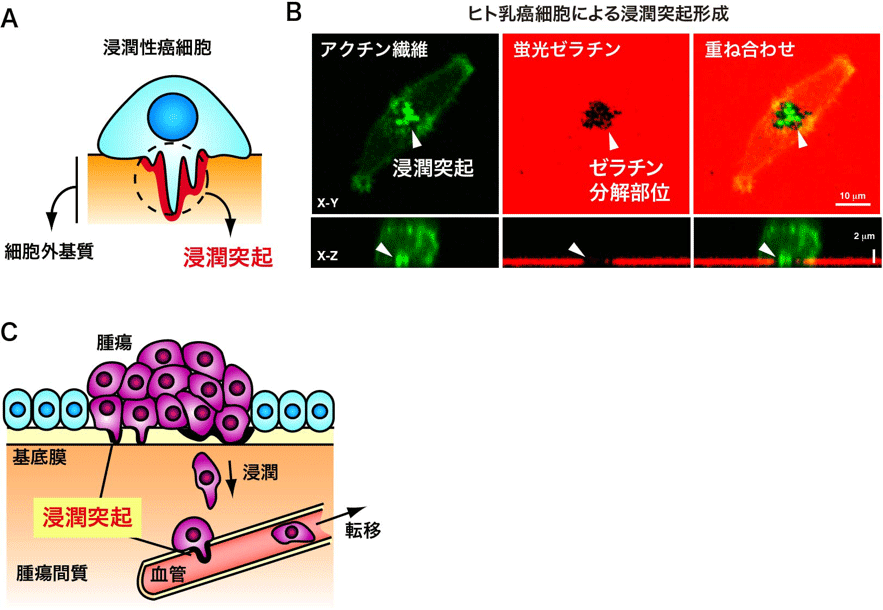
② スキルス胃がん腹膜播種性転移の分子機構の解明
スキルス胃がんは明らかな腫瘤を形成しないため早期発見が困難であり、非常に進展が早く、高頻度に腹膜播種性転移をきたす予後不良の難治がんです。私たちはスキルス胃がんの特性を制御する分子機構の解明を目的として研究を行っています。これまでにスキルス胃がんで特異的に活性化されるシグナル伝達分子を複数同定しており、現在これらの分子について腹膜播種性転移における機能と治療標的としての有用性を検討しています。またスキルス胃がんでは高度に間質増生が起こるため、スキルス胃がん細胞と間質線維芽細胞との相互作用など腫瘍微小環境の影響について解析を行っています(図2)。さらに、スキルス胃がん細胞を遺伝学的にマルチカラー蛍光ラベルして、腹膜播種巣における腫瘍不均一性のイメージング解析なども行なっています(図3)。これらの研究によりスキルス胃がんの進展機構を明らかにし、画期的な治療法の開発につなげたいと考えています。
研究業績
過去5年間の発表論文
- Yamaguchi H and Miyazaki M: Cell biology of cancer peritoneal metastasis: multiclonal seeding and peritoneal tumor microenvironment. Cancer Science 116: 1171-1180, 2025.
- Yamaguchi H and Miyazaki M: Heterocellular Adhesion in Cancer Invasion and Metastasis: Interactions between Cancer Cells and Cancer-Associated Fibroblasts. Cancers 16: 1636553, 2024.
- Miyazaki M, Nakabo A, Nagano Y, Nagamura Y, Yanagihara K, Ohki R, Nakamura Y, Fukami K, Kawamoto J, Umayahara K, Sakamoto M, Iwaya K, Yamaguchi H: Tissue factor-induced fibrinogenesis mediates cancer cell clustering and multiclonal peritoneal metastasis. Cancer Letters 553: 215983, 2023.
- Yamaguchi H, Nagamura Y, Miyazaki M: Receptor tyrosine kinases amplified in diffuse-type gastric carcinoma: potential targeted therapies and novel downstream effectors. Cancers 14: 3750, 2022.
- Shirakihara T, Yamaguchi H, Kondo T, Yashiro M, Sakai R: Transferrin receptor 1 promotes the fibroblast growth factor receptor-mediated oncogenic potential of diffused-type gastric cancer. Oncogene 41: 2587-2596, 2022.
- Miyamoto S, Nagano Y, Miyazaki M, Nagamura Y, Sasaki K, Kawamura T, Yanagihara K, Imai T, Ohki R, Yashiro M, Tanaka M, Sakai R, and Yamaguchi H: Integrin α5 mediates cancer cell-fibroblast adhesion and peritoneal dissemination of diffuse-type gastric carcinoma. Cancer Letters 526: 335-345 2022.
- Nagamura Y, Miyazaki M, Nagano Y, Tomiyama A, Ohki R, Yanagihara K, Sakai R, and Yamaguchi H: SHP2 as a potential therapeutic target in diffuse-type gastric carcinoma addicted to receptor tyrosine kinase signaling. Cancers 13: 4309, 2021.
- Nagamura Y, Miyazaki M, Nagano Y, Yuki M, Fukami K, Yanagihara K, Sasaki K, Sakai R, and Yamaguchi H: PLEKHA5 regulates the survival and peritoneal dissemination of diffuse-type gastric carcinoma cells with Met gene amplification. Oncogenesis 10: 25, 2021.
- Nakano Y, Takadera M, Miyazaki M, Qiao Z, Nakajima K, Noguchi R, Oyama R, Kimura Y, Okuhiro Y, Yamasaki K, Kunihiro N, Fukushima H, Inoue T, Hara J, Ozawa T, Kondo T, and Ichimura K: Drug screening with a novel tumor-derived cell line identified alternative therapeutic options for patients with atypical teratoid/rhabdoid tumor. Human Cell 34: 271-278, 2021
- Kobayashi T, Miyazaki M, Sasaki N, Yamamuro S, Uchida E, Kawauchi D, Takahashi M, Otsuka Y, Kumagai K, Takeuchi S, Toyooka T, Otani N, Wada K, Narita Y, Yamaguchi H, Muragaki Y, Kawamata T, Mori K, Ichimura K and Tomiyama A: Enhanced malignant phenotypes of glioblastoma cells surviving NPe6-mediated photodynamic therapy are regulated via ERK1/2 activation. Cancers 12: 3641, 2020
- Yoneda A, Kanemaru K, Matsubara A, Takai E, Shimozawa M, Yanagihara K, Satow R, Yamaguchi H, Nakamura Y, and Fukami K: Phosphatidylinositol 4,5-bisphosphate is localized in the outer leaflet of the plasma membrane and regulates cell adhesion and motility. Biochem. Biophys. Res. Comm. 527: 1050-1056, 2020.
それ以前の代表的な発表論文
- Miyagawa T, Hasegawa K, Aoki Y, Watanabe T, Otagiri Y, Arasaki K, Wakana Y, Asano K, Tanaka M, Yamaguchi H, Tagaya M, and Inoue H: MT1-MMP recruits the ER-Golgi SNARE Bet1 for efficient MT1-MMP transport to the plasma membrane. Journal of Cell Biology 218: 3355-3371, 2019.
- Miyamoto S, Narita T, Komiya M, Fujii G, Hamoya T, Nakanishi R, Tamura S, Kurokawa T, Takahashi M, and Mutoh M: Novel screening system revealed that intracellular cholesterol trafficking can be a good target for colon cancer prevention. Scientific Reports 9: 6192, 2019.
- Miyamoto S, Nagamura Y, Nakabo A, Okabe A, Yanagihara K, Fukami K, Sakai R, and Yamaguchi H: Aberrant alternative splicing of RHOA is associated with loss of its expression and activity in diffuse-type gastric carcinoma cells. Biochem. Biophys. Res. Comm. 495: 1942-1947, 2018.
- Yamaguchi H, Ito Y, Miura N, Nagamura Y, Nakabo A, Fukami K, Honda K, and Sakai R: Actinin-1 and actinin-4 play essential but distinct roles in invadopodia formation by carcinoma cells. Eur. J. Cell Biol. 96: 685-694, 2017.
- Tomiyama A, Uekita T, Kamata R, Sasaki K, Takita J, Ohira M, Nakagawara A, Kitanaka C, Mori K, Yamaguchi H, and Sakai R: Flotillin-1 regulates oncogenic signaling in neuroblastoma through receptor endocytosis of anaplastic lymphoma kinase. Cancer Res. 74: 3790-3801, 2014.
- Otsubo C, Otomo R, Miyazaki M, Matsushima-Hibiya Y, Kohno T, Iwakawa R, Takeshita F, Okayama H, Ichikawa H, Saya H, Kiyono T, Ochiya T, Tashiro F, Nakagama H, Yokota J, Enari M: TSPAN2 Is Involved in Cell Invasion and Motility during Lung Cancer Progression. Cell Rep. 7: 527-538, 2014.
- Otomo R, Otsubo C, Matsushima-Hibiya Y, Miyazaki M, Tashiro F, Ichikawa H, Kohno T, Yokota J, Nakagama H, Taya Y, Enari M: TSPAN12 is a critical factor for cancer-fibroblast cell contact-mediated cancer invasion. Proc. Natl. Acad. Sci. USA 111: 18691-18696, 2014.
- Ohata H, Miyazaki M, Otomo R, Matsushima-Hibiya Y, Otsubo C, Nagase T, Arakawa H, Yokota J, Nakagama H, Taya Y, Enari M: NuMA Is Required for the Selective Induction of p53 Target Genes. Mol. Cell. Biol. 33: 2447-2457, 2013.
- Kanemaru K*, Nakamura Y*, Sato K, Takahashi S, Yamaguchi M, Ichinohe M, Kiyonari H, Shioi G, Asagiri M, Jamora C, Kouchi Z, Yamaguchi H, and Fukami K: Epidermal loss of phospholipase C δ1 results in myeloproliferation in mice. Nature Commun. 3: 963, 2012.
- Yamaguchi H, Yoshida S, Muroi E, Yoshida N, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, and Fukami K: PI3-kinase signaling pathway mediated by p110α regulates invadopodia formation. J. Cell Biol. 193: 1275-1288, 2011.
- Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, and Fukami K: Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 69: 8594-8602, 2009.
- Oser M, Yamaguchi H, Mader CC, Arias M, DesMarais V, van Rheenen J, Koleske AJ, and Condeelis J: Cortactin regulates cofilin and N-WASP activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186: 571-587, 2009.
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, and Gertler FB: A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 15: 813-828, 2008.
- Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, and Condeelis J: Molecular mechanisms of invadopodium formation: The role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 168: 441-452, 2005.
- Oikawa T, Yamaguchi H, Itoh T, Kato M, Ijuin T, Yamazaki D, Suetsugu S, and Takenawa T: PtIns(3,4,5)P3 binding is necessary for WAVE2-induced formation of lamellipodia. Nature Cell Biol. 6: 420-426, 2004.
- Lorenz M, Yamaguchi H, Wang Y, Singer RH, and Condeelis JS: Imaging sites of N-WASP activity in lamellipodia and invadopodia of carcinoma cells. Curr. Biol. 14: 697-703, 2004.
- Yamaguchi H, Miki H, and Takenawa T: Neural Wiskott-Aldrich syndrome protein is involved in hepatocyte growth factor-induced migration, invasion, and tubulogenesis of epithelial cells. Cancer Res. 62: 2503-2509, 2002.
- Martinez-Quiles N, Rohatgi R, Anton IM, Medina M, Saville SP, Miki H, Yamaguchi H, Takenawa T, Hartwig JH, Geha RS, and Ramesh N: WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nature Cell Biol. 3: 484-491, 2001.
- Miki H, Yamaguchi H, Suetsugu S, and Takenawa T: IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408: 732-735, 2000.
- Yamaguchi H, Miki H, Suetsugu S, Ma L, Kirschner MW, and Takenawa T: Two tandem verprolin homology domains are necessary for a strong activation of Arp2/3 complex-induced actin polymerization and induction of microspike formation by N-WASP. Proc. Natl. Acad. Sci. USA 97: 12631-12636, 2000.