Department of Cancer Cell Research
Member
You can scroll horizontally.
| Chief (Principal Investigator) | Hideki Yamaguchi, Ph.D. | Researchmap/Google Scholar e-mail: h-yamaguchi |
| Research Scientist | ||
| Research Assistant | Yuko Nagamura | |
| Visiting Scientist | Makoto Miyazaki, Ph.D. | Chiba Cancer Center Research Institute Researchmap/Google Scholar |
| Visiting Scientist | Benio Tsuchiya, Ph.D. | Researchmap |
| Research Student | Hinako Fujii | Tokyo College of Biotechnology |
| Research Student | Hiroto Honmoto | Nihon University School of Pharmacy |
*Please add @po.kyoundo.jp after e-mail address
Overview
Cancer has been the leading cause of death in Japan. Metastasis, the spread of cancer to other parts of the body, accounts for about 90% of cancer deaths. Therefore, the control of metastasis is one of the most important issues in the treatment of cancer. However, as the molecular mechanisms regulating metastasis remain incompletely understood, further biological analyses are necessary for deeper understanding to develop anti-metastasis therapy. We aim to understand the role of cellular structures called invadopodia that play important roles in cancer metastasis. We also intend to reveal the mechanisms underlying peritoneal metastasis of scirrhous gastric carcinoma, an aggressive and refractory subtype of gastric carcinoma whose incidence is high in Japan. Our research should provide important insights into the molecular basis of cancer metastasis and lead to the development of novel cancer therapeutics targeting metastasis.Projects
1. Molecular mechanisms of invadopodia formation and their roles in blood-borne metastasis
Invadopodia are membrane protrusions formed by invasive cancer cells. Invadopodia are rich in actin filaments and matrix metalloproteases with the ability to degrade extracellular matrix (Figs. 1A and B). So far, we have identified several structural and regulatory components of invadopodia and elucidated their functions in invadopodia formation. Moreover, our approach demonstrated that invadopodia play a pivotal role in invasion and hematogenous metastasis of breast cancer (Fig. 1C). We have recently identified several novel components of invadopodia and undertaken their detailed characterization to clarify the molecular mechanisms of invadopodia formation. We are also seeking novel functional molecules and inhibitory compounds by high-throughput screening of gene and chemical libraries, respectively, using invadopodia formation as a readout.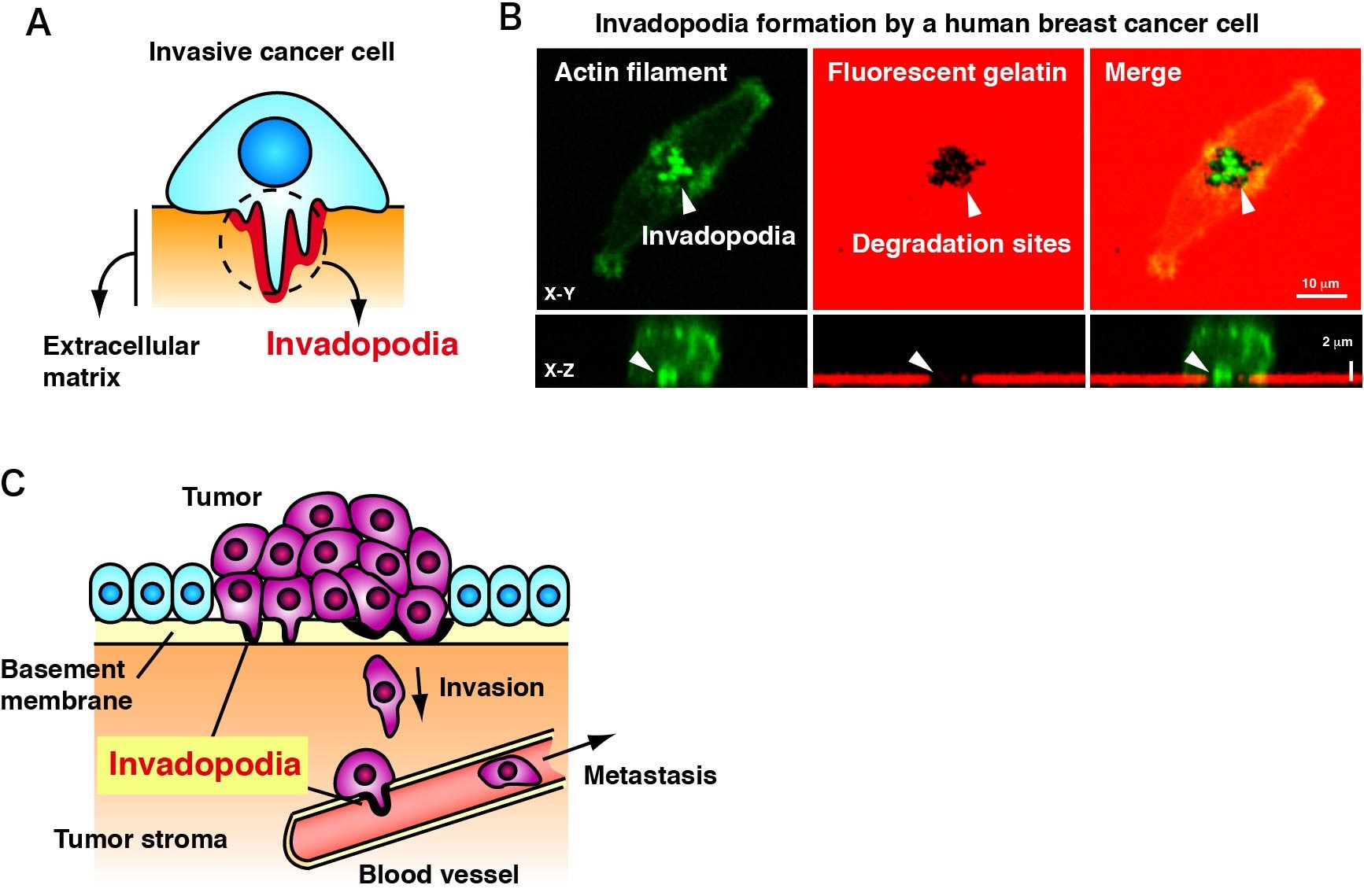
2. Molecular mechanisms of peritoneal dissemination of scirrhous gastric carcinoma
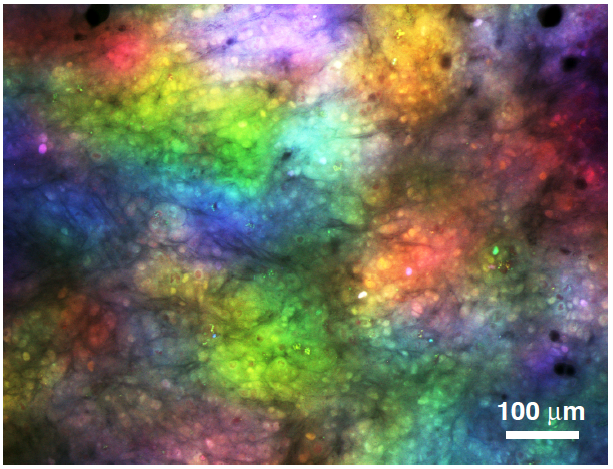
Scirrhous gastric carcinoma is a refractory carcinoma with poor prognosis owing to its difficulty in early detection, rapid infiltrative growth, and frequent peritoneal dissemination. We are investigating molecular mechanisms governing the aggressive phenotypes of scirrhous gastric carcinoma. We identified several signaling pathways and molecules specifically activated in scirrhous gastric carcinoma. Their roles in peritoneal dissemination and potentials as therapeutic targets are under investigation. Since scirrhous gastric carcinoma is associated with massive fibrosis, we also assess the involvement of tumor microenvironment, including interaction between carcinoma cells and stromal fibroblasts (Fig. 2). Moreover, we make an attempt to visualize tumor clonality and heterogeneity of peritoneally disseminated tumors by multi-color fluorescent imaging (Fig. 3). Our work will contribute to elucidation of the molecular mechanisms underlying progression of scirrhous gastric carcinoma and lead to the development of innovative cancer therapeutics.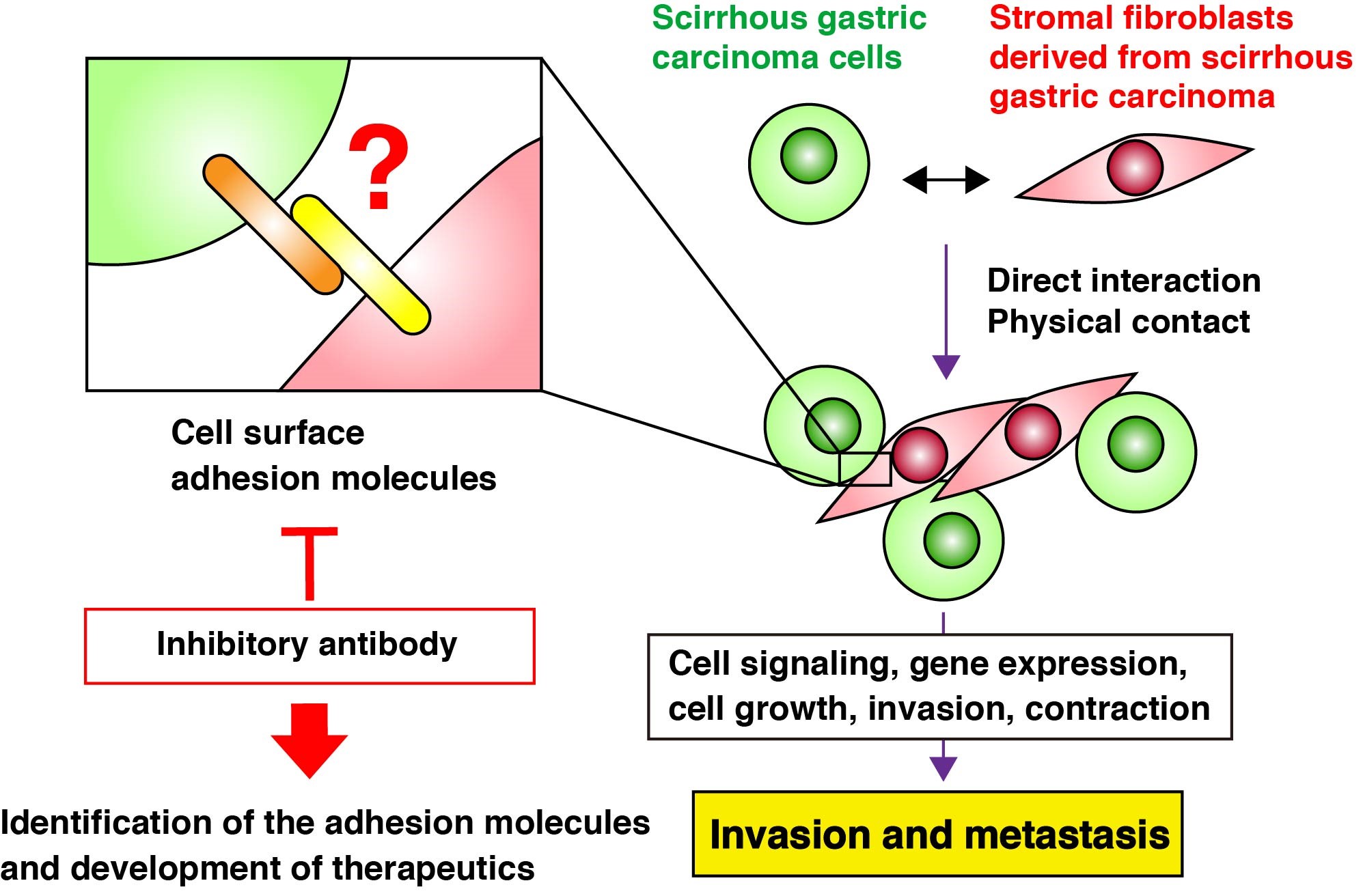
Publications
Last 5 years
- Yamaguchi H and Miyazaki M: Heterocellular Adhesion in Cancer Invasion and Metastasis: Interactions between Cancer Cells and Cancer-Associated Fibroblasts. Cancers 16: 1636553, 2024.
- Miyazaki M, Nakabo A, Nagano Y, Nagamura Y, Yanagihara K, Ohki R, Nakamura Y, Fukami K, Kawamoto J, Umayahara K, Sakamoto M, Iwaya K, Yamaguchi H: Tissue factor-induced fibrinogenesis mediates cancer cell clustering and multiclonal peritoneal metastasis. Cancer Letters 553: 215983, 2023.
- Yamaguchi H, Nagamura Y, Miyazaki M: Receptor tyrosine kinases amplified in diffuse-type gastric carcinoma: potential targeted therapies and novel downstream effectors. Cancers 14: 3750, 2022.
- Shirakihara T, Yamaguchi H, Kondo T, Yashiro M, Sakai R: Transferrin receptor 1 promotes the fibroblast growth factor receptor-mediated oncogenic potential of diffused-type gastric cancer. Oncogene 41: 2587-2596, 2022.
- Miyamoto S, Nagano Y, Miyazaki M, Nagamura Y, Sasaki K, Kawamura T, Yanagihara K, Imai T, Ohki R, Yashiro M, Tanaka M, Sakai R, and Yamaguchi H: Integrin α5 mediates cancer cell-fibroblast adhesion and peritoneal dissemination of diffuse-type gastric carcinoma. Cancer Letters 526: 335-345 2022.
- Nagamura Y, Miyazaki M, Nagano Y, Tomiyama A, Ohki R, Yanagihara K, Sakai R, and Yamaguchi H: SHP2 as a potential therapeutic target in diffuse-type gastric carcinoma addicted to receptor tyrosine kinase signaling. Cancers 13: 4309, 2021.
- Nagamura Y, Miyazaki M, Nagano Y, Yuki M, Fukami K, Yanagihara K, Sasaki K, Sakai R, and Yamaguchi H: PLEKHA5 regulates the survival and peritoneal dissemination of diffuse-type gastric carcinoma cells with Met gene amplification. Oncogenesis 10: 25, 2021.
- Nakano Y, Takadera M, Miyazaki M, Qiao Z, Nakajima K, Noguchi R, Oyama R, Kimura Y, Okuhiro Y, Yamasaki K, Kunihiro N, Fukushima H, Inoue T, Hara J, Ozawa T, Kondo T, and Ichimura K: Drug screening with a novel tumor-derived cell line identified alternative therapeutic options for patients with atypical teratoid/rhabdoid tumor. Human Cell 34: 271-278, 2021
- Kobayashi T, Miyazaki M, Sasaki N, Yamamuro S, Uchida E, Kawauchi D, Takahashi M, Otsuka Y, Kumagai K, Takeuchi S, Toyooka T, Otani N, Wada K, Narita Y, Yamaguchi H, Muragaki Y, Kawamata T, Mori K, Ichimura K and Tomiyama A: Enhanced malignant phenotypes of glioblastoma cells surviving NPe6-mediated photodynamic therapy are regulated via ERK1/2 activation. Cancers 12: 3641, 2020
- Yoneda A, Kanemaru K, Matsubara A, Takai E, Shimozawa M, Yanagihara K, Satow R, Yamaguchi H, Nakamura Y, and Fukami K: Phosphatidylinositol 4,5-bisphosphate is localized in the outer leaflet of the plasma membrane and regulates cell adhesion and motility. Biochem. Biophys. Res. Comm. 527: 1050-1056, 2020.
- Miyagawa T, Hasegawa K, Aoki Y, Watanabe T, Otagiri Y, Arasaki K, Wakana Y, Asano K, Tanaka M, Yamaguchi H, Tagaya M, and Inoue H: MT1-MMP recruits the ER-Golgi SNARE Bet1 for efficient MT1-MMP transport to the plasma membrane. Journal of Cell Biology 218: 3355-3371, 2019.
- Miyamoto S, Narita T, Komiya M, Fujii G, Hamoya T, Nakanishi R, Tamura S, Kurokawa T, Takahashi M, and Mutoh M: Novel screening system revealed that intracellular cholesterol trafficking can be a good target for colon cancer prevention. Scientific Reports 9: 6192, 2019.
Before 2019 (selected)
- Miyamoto S, Nagamura Y, Nakabo A, Okabe A, Yanagihara K, Fukami K, Sakai R, and Yamaguchi H: Aberrant alternative splicing of RHOA is associated with loss of its expression and activity in diffuse-type gastric carcinoma cells. Biochem. Biophys. Res. Comm. 495: 1942-1947, 2018.
- Yamaguchi H, Ito Y, Miura N, Nagamura Y, Nakabo A, Fukami K, Honda K, and Sakai R: Actinin-1 and actinin-4 play essential but distinct roles in invadopodia formation by carcinoma cells. Eur. J. Cell Biol. 96: 685-694, 2017.
- Tomiyama A, Uekita T, Kamata R, Sasaki K, Takita J, Ohira M, Nakagawara A, Kitanaka C, Mori K, Yamaguchi H, and Sakai R: Flotillin-1 regulates oncogenic signaling in neuroblastoma through receptor endocytosis of anaplastic lymphoma kinase. Cancer Res. 74: 3790-3801, 2014.
- Otsubo C, Otomo R, Miyazaki M, Matsushima-Hibiya Y, Kohno T, Iwakawa R, Takeshita F, Okayama H, Ichikawa H, Saya H, Kiyono T, Ochiya T, Tashiro F, Nakagama H, Yokota J, Enari M: TSPAN2 Is Involved in Cell Invasion and Motility during Lung Cancer Progression. Cell Rep. 7: 527-538, 2014.
- Otomo R, Otsubo C, Matsushima-Hibiya Y, Miyazaki M, Tashiro F, Ichikawa H, Kohno T, Yokota J, Nakagama H, Taya Y, Enari M: TSPAN12 is a critical factor for cancer-fibroblast cell contact-mediated cancer invasion. Proc. Natl. Acad. Sci. USA 111: 18691-18696, 2014.
- Ohata H, Miyazaki M, Otomo R, Matsushima-Hibiya Y, Otsubo C, Nagase T, Arakawa H, Yokota J, Nakagama H, Taya Y, Enari M: NuMA Is Required for the Selective Induction of p53 Target Genes. Mol. Cell. Biol. 33: 2447-2457, 2013.
- Kanemaru K*, Nakamura Y*, Sato K, Takahashi S, Yamaguchi M, Ichinohe M, Kiyonari H, Shioi G, Asagiri M, Jamora C, Kouchi Z, Yamaguchi H, and Fukami K: Epidermal loss of phospholipase C δ1 results in myeloproliferation in mice. Nature Commun. 3: 963, 2012.
- Yamaguchi H, Yoshida S, Muroi E, Yoshida N, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, and Fukami K: PI3-kinase signaling pathway mediated by p110α regulates invadopodia formation. J. Cell Biol. 193: 1275-1288, 2011.
- Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, and Fukami K: Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 69: 8594-8602, 2009.
- Oser M, Yamaguchi H, Mader CC, Arias M, DesMarais V, van Rheenen J, Koleske AJ, and Condeelis J: Cortactin regulates cofilin and N-WASP activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186: 571-587, 2009.
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, and Gertler FB: A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 15: 813-828, 2008.
- Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, and Condeelis J: Molecular mechanisms of invadopodium formation: The role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 168: 441-452, 2005.
- Oikawa T, Yamaguchi H, Itoh T, Kato M, Ijuin T, Yamazaki D, Suetsugu S, and Takenawa T: PtIns(3,4,5)P3 binding is necessary for WAVE2-induced formation of lamellipodia. Nature Cell Biol. 6: 420-426, 2004.
- Lorenz M, Yamaguchi H, Wang Y, Singer RH, and Condeelis JS: Imaging sites of N-WASP activity in lamellipodia and invadopodia of carcinoma cells. Curr. Biol. 14: 697-703, 2004.
- Yamaguchi H, Miki H, and Takenawa T: Neural Wiskott-Aldrich syndrome protein is involved in hepatocyte growth factor-induced migration, invasion, and tubulogenesis of epithelial cells. Cancer Res. 62: 2503-2509, 2002.
- Martinez-Quiles N, Rohatgi R, Anton IM, Medina M, Saville SP, Miki H, Yamaguchi H, Takenawa T, Hartwig JH, Geha RS, and Ramesh N: WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nature Cell Biol. 3: 484-491, 2001.
- Miki H, Yamaguchi H, Suetsugu S, and Takenawa T: IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408: 732-735, 2000.
- Yamaguchi H, Miki H, Suetsugu S, Ma L, Kirschner MW, and Takenawa T: Two tandem verprolin homology domains are necessary for a strong activation of Arp2/3 complex-induced actin polymerization and induction of microspike formation by N-WASP. Proc. Natl. Acad. Sci. USA 97: 12631-12636, 2000.